Brian McEvoy B.Sc. MBA1; Hervé Michel B.Sc.2; Daniel Howell B.Sc.3, Philip Roxby B.Eng.4
1 Senior Director Global Technologies, STERIS, IDA Business & Technology Park, Tullamore, County Offaly, R35 X865, Ireland
2 Director, Radiation Technology EMEAA, STERIS, Hogenweidstrasse 6, 4658 Däniken, Switzerland
3 European RTC & UK Laboratory Operations Manager, STERIS, Roydsdale Way, Euroway Trading Estate, Bradford, W. Yorkshire, BD4 6SE, UK
4 Technical Services Manager, STERIS, 15-16E Mervue Business Park, Galway, H91 D3T0, Ireland
Abstract
Following years of discussions and debate regarding the economics of X-ray radiation for sterilization of healthcare products, the benefits of the technology are now being realized. X-ray, like gamma radiation, is a process whereby energic photons penetrate to sterilize medical devices. Compared to gamma, photons in the bremsstrahlung spectrum from X-ray radiation allow for improved dose uniformity ratio, higher dose rates, and shorter process time, which provide additional opportunities for sterilization process enhancement. Such improvements may be realized in a number of ways: 1) economic, where more products may be processed on a carrier; 2) improved dose range fit; and/or 3) wider material compatibility. Despite noted benefits, X-ray sterilization has not yet been widely accepted and currently accounts for less than 5% of the contract sterilization market. This paper brings X-ray sterilization into focus by sharing knowledge and experience gained over the past 10 years at STERIS Däniken site with an aim to identify opportunities for future medical device sterilization.
Introduction
Radiation sterilization of medical products was first explored in the late 1940’s, with production processing commencing in 1957 by Ethicon, Inc using a 7MeV 5kW (E-beam). By the mid 1960’s, the use of cobalt-60 radiation sources was prevalent.[2] By the mid 1970’s, the use of E-beam was again explored as an alternative to isotope processing, and in 2017 E-beam accounted for 15% of medical device processing in the U.S.[3] Today, while a significant reliance remains on gamma, a pressing need for a viable alternative is recognized to address concerns regarding cobalt supply and radioactive source security.3 One of the key advantages to gamma irradiation is that it is highly effective at treating a wide variety of products and package configurations with varying densities. Therefore, a viable alternative should also demonstrate similar characteristics. Alternatives to isotope radiation come in the form of accelerator-based radiation, such as E-beam and X-ray. With a long history of use, E-beam is an excellent modality choice but is limited by the depth of penetration of the electrons, and does not always offer a suitable alternative to large-volume, cobalt-based processes. On the other hand, high-energy X-rays are more penetrating (than E-beam) and therefore offer a viable alternative to gamma radiation.[4] When considering a change of the sterilization source, e.g., from gamma to X-ray, key processing parameters such as temperature, dose rate, incremental dose process, maximum dose, etc. must be evaluated, as they may differ significantly. Therefore, a better understanding of the X-ray irradiation process for sterilization of medical devices could create wider acceptance of the technology and facilitate transitioning from gamma to X-ray. The aim of this paper is to share experience from the first large-scale medical device X-ray sterilization at STERIS Däniken (Switzerland). The experience gained processing a wide array of materials and devices over the past 10 years can help inform potential users of the benefits and opportunities of X-ray processing.
Opportunities with X-ray
Photon-to-Photon Technology
From the early work of Wilhelm Roentgen in 1895 at his laboratory in University of Würzburg, X-rays have been widely applied in medical and industrial diagnostic instruments because of their unique properties.[5] Bremsstrahlung X-rays are emitted when energic electrons generated by an E-beam source strike a target material and are deflected by the atomic nuclei in the material. The X-ray intensity increases with the electron beam current, the kinetic energy of the electrons, and the atomic number of the target material.1 In healthcare sterilization, radiation energies in the range of 3–7 MeV and beam powers in the range of 100–700 kW are needed to provide an alternative to gamma irradiation. These elevated power levels must be used to compensate for the inefficiency of the conversion from electron to photon: Typical conversion efficiency for a 7 MeV machine using tantalum target material would be approximately 12%.1,[6] While X-ray suffers from conversion efficiency, the angular distribution of X-rays in the forward direction versus the isotropic field of gamma, coupled with higher dose rates, compensate to make X-ray a viable alternative.1 In the context of equipment and facility design with regard to accelerator-based technology, there is ample documentation in the literature,1,3,4,6 and X-ray equipment configurations are currently available from a number of suppliers including CGN Dasheng, IBA , and Mevex.
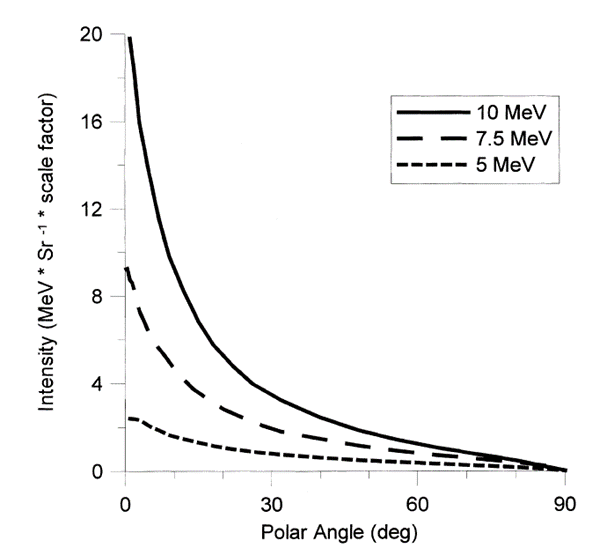
One of the benefits of transitioning from gamma radiation to X-ray is that with both sources, photons are used to deliver the dose to product. However, gamma and X-ray processes produce those photons in different ways: Cobalt-60 decays into a stable Nickel-60 isotope and emits two wavelengths of high-energy gamma-rays (1.17 and 1.33 MeV),[7] whereas X-rays are produced by striking a metal target with the electron beam from an accelerator.1 Advantageously, in an X-ray radiation field, the majority of photons propagate from the converter in the same general direction as the incident electrons. As shown in Figure 2, the intensity of the X-ray photons increases with the energy and when the polar angle decreases.1 The directionality of the photons perpendicular to the product surface has the effect of optimizing the photon capture rate relative to the more isotropic gamma rays from Cobalt-60.
Figure 1: Energy spectra of X-ray photons generated by bremsstrahlung on a tantalum target with electron of 5 and 7 MeV (right) in comparison with gamma energy spectrum (left). Adapted from Meissner et al.7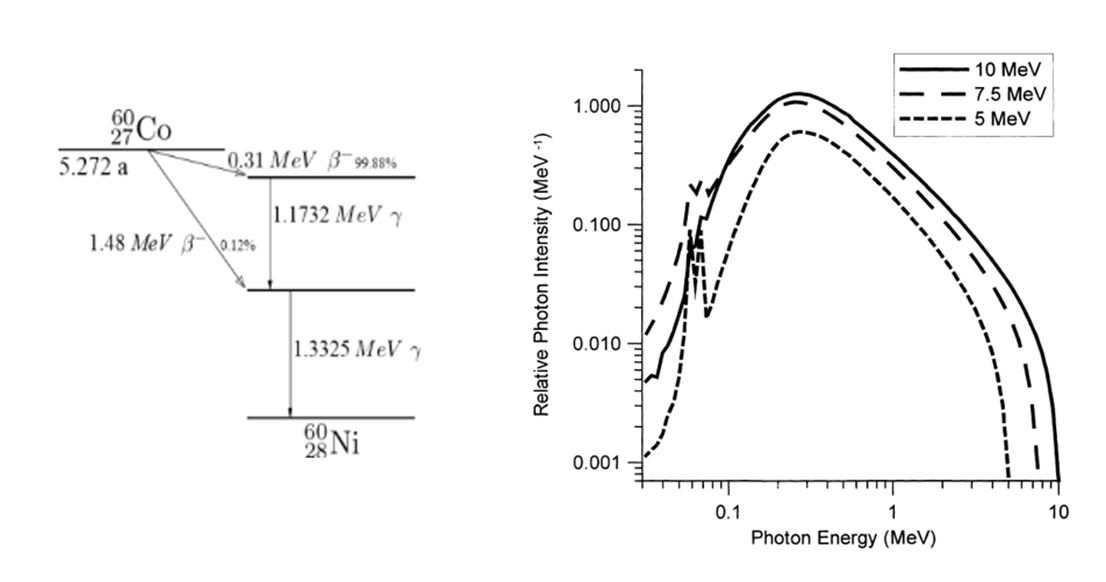
Figure 2: Photons intensity as a function of angle at 5, 7.5, and 10 MeV incident electron energies. Adapted from Adapted from Meissner et al.7
 Temperature
Temperature
Temperature has a significant effect on polymeric material properties, often observed with accelerated aging studies. Excess energy from radiation processing increases the temperature of the treated material. However, the mechanism by which heat is imparted varies by radiation process. The importance of the radiation field is highlighted when one examines temperature differences experienced by products treated with both gamma and X-ray.
Because of the directionality of the photons in X-ray radiation processes and the design of the target used to convert the electron to photon, the X-ray radiation field surface is smaller than the radiation field resulting from a gamma source rack. Therefore, the dose is efficiently delivered to products only when they are in front of the X-ray target. In gamma processes, products receive dose from the time they enter the process radiation chamber until the time they exit. Furthermore, an incremental or multiple-pass process versus continuous process may also have an influence on how the dose is delivered, and subsequently how product properties may be impacted. High-power X-ray irradiators are often an incremental design, which means that the total dose is given in multiple passes in front of the X-ray source. As a consequence, the product will be exposed to different temperatures than in a gamma process, where the product spends a few hours in the radiation room, usually at high temperatures (>45°C). Additionally, a temperature rise associated with the total dose received will also impact the temperature of the product. For example, a comparison made at the Däniken facility between a gamma irradiator (average dose rate ~ 3 kGy/h) and an X-ray irradiator (; average dose rate ~ 250 kGy/h) demonstrated that the difference between the start and end temperature is approximately 22°C and 11°C respectively, at similar maximum dose. This 11°C difference could be significant with regard to influence on materials properties. For example, if one considers the accelerated aging of sterile barrier systems for medical devices, the Arrhenius equation demonstrates a Q10 value of 2 (for every 10°C increase in temperature, the rate of accelerated aging doubles) for temperature.[9] If Q10 is applied to the observed 11°C increase in gamma vs. X-ray, it follows that the materials potentially age twice as fast in gamma vs. X-ray.
Dose Rate
The dose rate is the quantity of radiation absorbed per unit time. In radiation processing, it is usually given in kGy/h or kGy/s and is related with the power of the irradiator or source. Therefore, comparisons of X-ray and gamma dose rate must be assessed on equivalent throughput systems. An X-ray irradiator dose rate may vary significantly depending on the irradiator design (e.g., beam current, converter design, and distance to product). It can be much higher than a dose rate in a gamma irradiator (a few hundred kGy/h) for high-power irradiators or irradiators with a smaller irradiation field, or lower for a low-power X-ray irradiator where dose rate is typically a few kGy/h.
As shown in Figure 3, higher dose rates can also limit the oxidative degradation of polymers by minimizing the time for oxygen replenishment required for radical-oxygen reactions. Consequently, as discussed in AAMI TIR17,[10] a material that is formerly qualified at a low dose rate (gamma) will typically require minimal qualification to demonstrate material compatibility at a higher dose rate, as the gamma qualification might be considered as the worst case scenario.
Figure 3: Dose rate effect on material properties.
Material Compatibility
One of the known aspects of radiation processing common to all three sources (Gamma, E-beam, and X-ray) is that the process may have a significant effect on the molecular structure of the processed material, which may lead to a modification of the medical device and/or its packaging integrity and properties. The influence of radiation-induced active chemical species on the properties and performance of a polymer is proportional to dose. When there is a distribution of dose within a part or within a product load, the resulting property changes can vary by location within the part, or from part to part. Often the most significant mode of radiation-induced degradation is the embrittling chain scission reaction that results from interaction with oxygen.10
Comparisons made at the STERIS Däniken facility between a gamma irradiator with a dose rate around 3 kGy/h and an X-ray irradiator (7 MeV, 560 kW) at a dose rate around 250 kGy/h on ultra high molecular weight polyethylene (UHMWPE) at five different doses (50, 75, 100, 125, and 150 kGy) tested in accordance with ASTM F2565,[11] demonstrate the impact on polymer properties, where X-ray was shown to be equivalent or better for some characteristics.[12] Preliminary accelerated aging studies performed on common packaging material at the same conditions revealed a reduced surface oxidation index for X-ray–irradiated packaging compared with gamma.
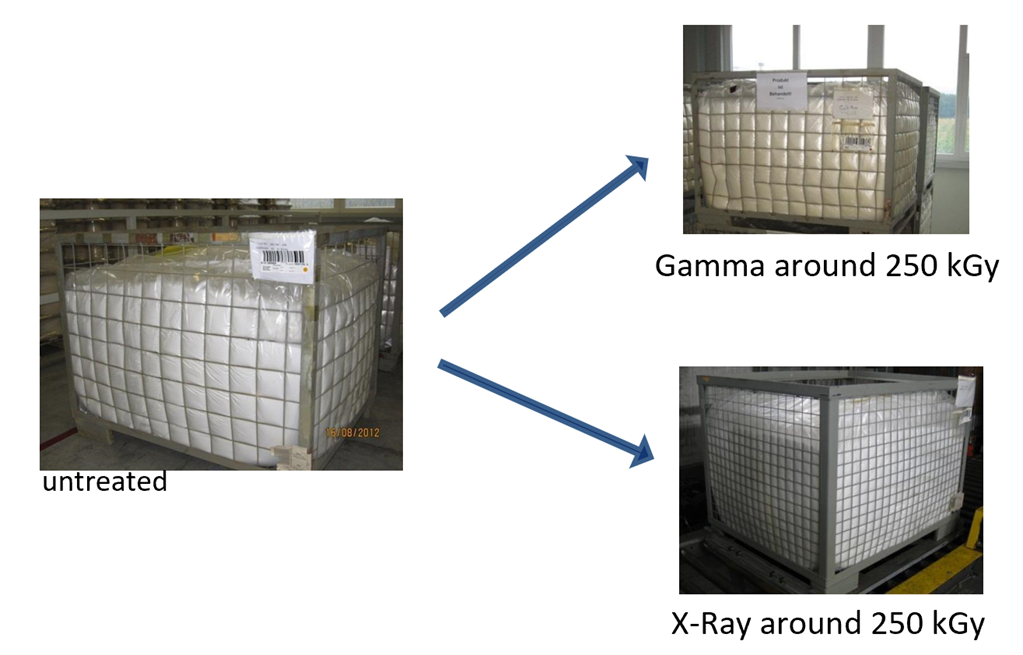
A well-known effect of the radiation process on material is color modification—where a number of polymers may discolor to yellow or brown following processing—as a consequence of the maximum dose received and possibly the dose rate. The degree of coloration is also dependent on the material and may potentially fade over time, and therefore while undesirable in a medical device, may be acceptable. Gamma sterilization is compatible with many materials. However, materials such as polyvinyl chloride, and polytetrafluoroethylene (PTFE) can be severely affected, rendering gamma sterilization unacceptable. As an example of the varying discoloration effect from differing radiation technologies, Figure 4 shows the discoloration effect of gamma and X-ray radiation (at same maximum dose) on PTFE. As demonstrated, significantly less discoloration is observed with PTFE processed with X-ray.
Figure 4: PTFE material coloration following processing with gamma and X-ray irradiation at the same maximum dose (circa. 250kGy).
Improved Dose Uniformity Ratio
The sterilization dose is the “minimum dose to achieve the specified requirements for sterility” and the maximum acceptable dose is the “highest dose that can be applied to a specified product without compromising safety, quality, or performance.”[13] In order for a radiation process to be feasible and suitable for the product, both the sterilization and maximum acceptable doses must be delivered.8
The dose uniformity ratio (DUR; defined as “ratio of the maximum to the minimum absorbed dose within the radiation container”13) the available dose window between the sterilization dose and the maximum acceptable dose. Therefore, the lower the DUR of the irradiation process, the greater the opportunity for efficient processing of products. A lower DUR for the same process load may result in improved product properties as the maximum dose delivered to the product will be reduced, therefore reducing deleterious effects such as oxidation and discoloration.
As a result of the X-ray photons having increased penetration and optimized photon energy delivery, improvements (over gamma) in DUR may be realized: As discussed in the Temperature section above, even with many lower-energy photons, bremsstrahlung photons from a 5.0-MeV E-beam penetrate slightly more than the radiation from a large-area source of gamma rays.4 This greater penetration is partly due to the higher energy photons in the bremsstrahlung spectrum and partly due to the angular distribution. X-ray systems have a directive beam of photons concentrated in the direction of the product, optimizing the photon capture rate. In contrast, the nearly isotropic radiation in an industrial gamma-ray facility has a wide angular distribution. Consequently, much of the gamma-ray emission is more divergent than a high-energy bremsstrahlung beam and enters the products at larger angles from the perpendicular direction. The outcome of this with regard to DUR performance is exemplified in the following examples observed at STERIS Däniken.
Operational Qualification Comparison
As shown in Figure 5, performance of operational qualification (OQ) of gamma and X-ray irradiators with identical product density and process load show a significant gain in DUR for X-ray. As an example of how to use this graphic, for a pallet of medical device of density 0.25 g/cm3, and a minimal dose of 25 kGy, the maximum dose received by the process load (pallet of 1.0m × 1.2m × 1.8m = 2.2m3) would be:
Gamma: 25 × 1.65 = ~ 42 kGy
X-ray: 25 × 1.45 = ~ 37 kGy
Figure 5: DUR as a function of density (g/cm3) for Däniken X-ray and gamma pallet irradiator.
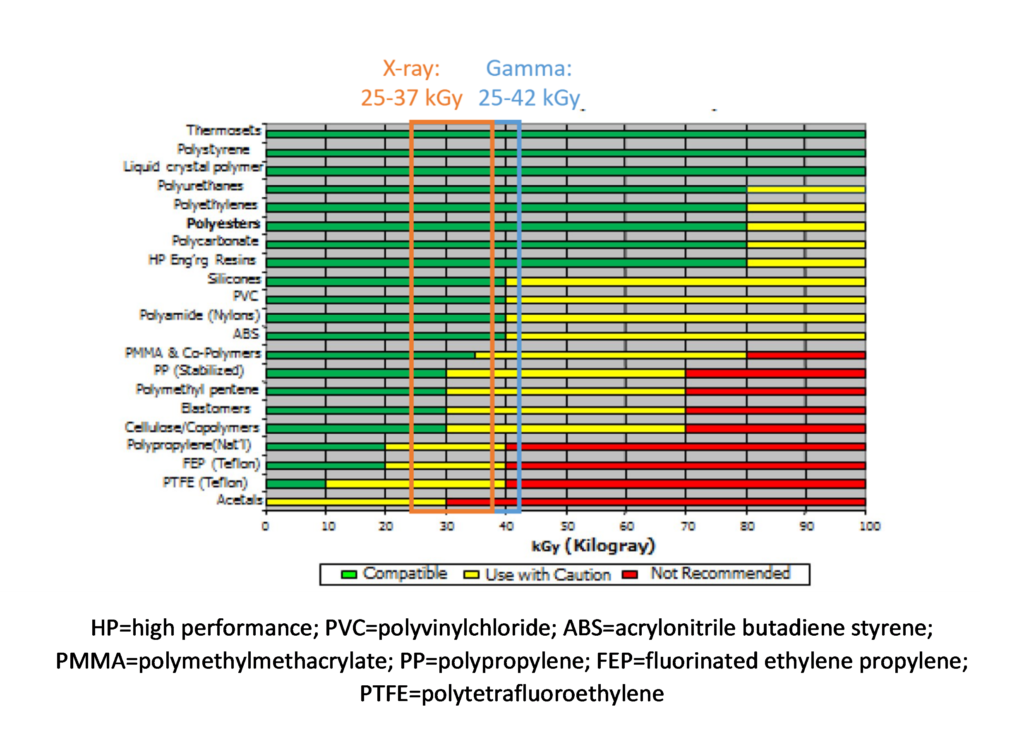
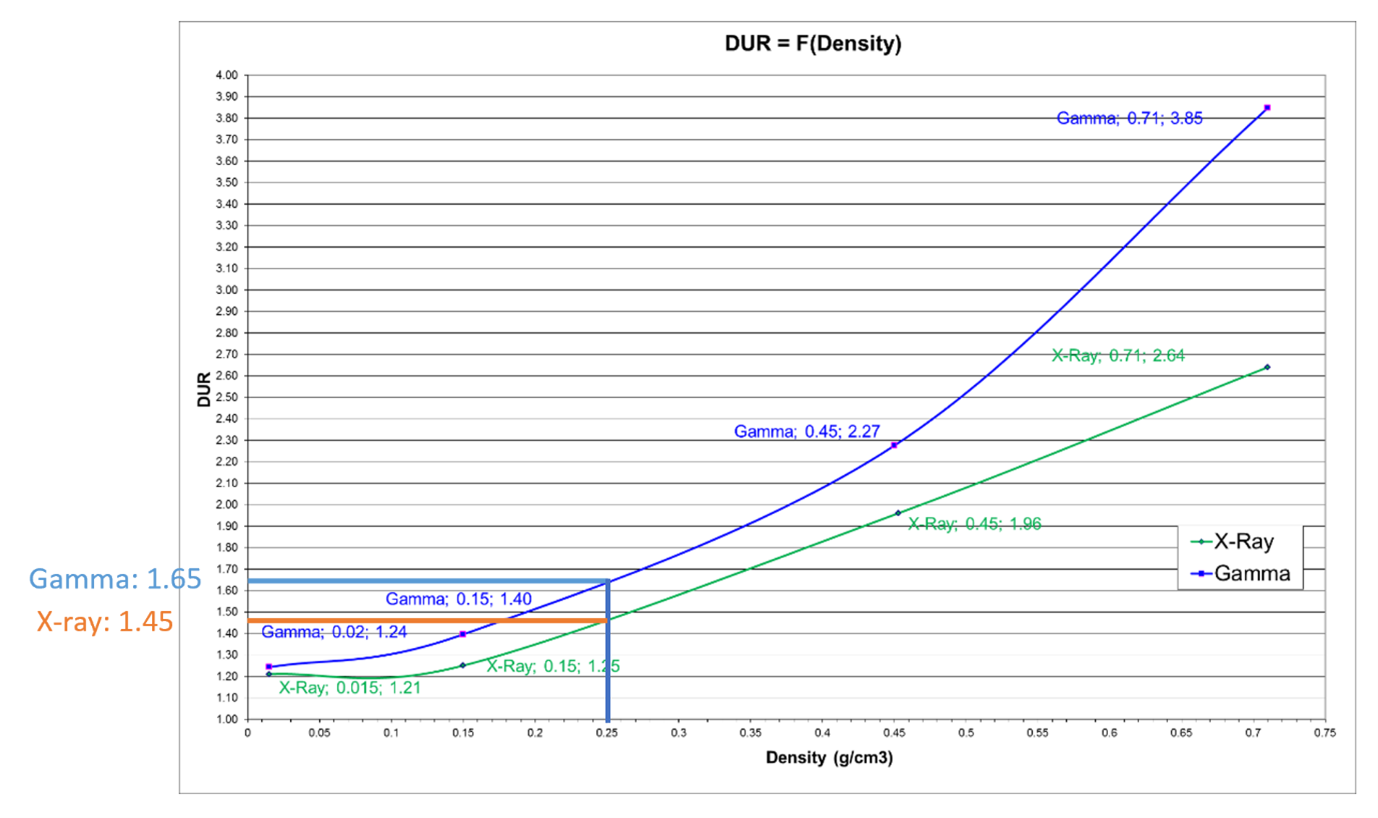 DUR is improved by 14% with X-ray. Such a reduction could be significant, especially with a polymer that might have marginal compatibility with radiation processing. Specifically, if one considers this example in conjunction with Figure 6, four polymers that would not be compatible with gamma potentially become available for X-ray. This is very much in line with expectations as summarized by Grégoire et al.[14] where “technical advantages of higher energies are better power utilization, reduced treatment time and improved dose uniformity” resulting in the benefits of throughput speed, reduced costs, and improved product quality.
DUR is improved by 14% with X-ray. Such a reduction could be significant, especially with a polymer that might have marginal compatibility with radiation processing. Specifically, if one considers this example in conjunction with Figure 6, four polymers that would not be compatible with gamma potentially become available for X-ray. This is very much in line with expectations as summarized by Grégoire et al.[14] where “technical advantages of higher energies are better power utilization, reduced treatment time and improved dose uniformity” resulting in the benefits of throughput speed, reduced costs, and improved product quality.
Figure 6: Relative radiation stability of medical polymer “families.” Adapted from AAMI TIR17.10
Performance Qualification Comparison
Improved DUR observed during OQ has been verified throughout performance qualification (PQ) studies on actual product configurations. Case studies below show the difference found on the same configuration when processing with gamma and X-ray at Däniken. These case studies demonstrate how an improvement in DUR can yield additional benefits to product processing, including more precise dose delivery, reduction in product temperature, and improved product throughput.
Case Study 1: Precise Dose for Product Testing
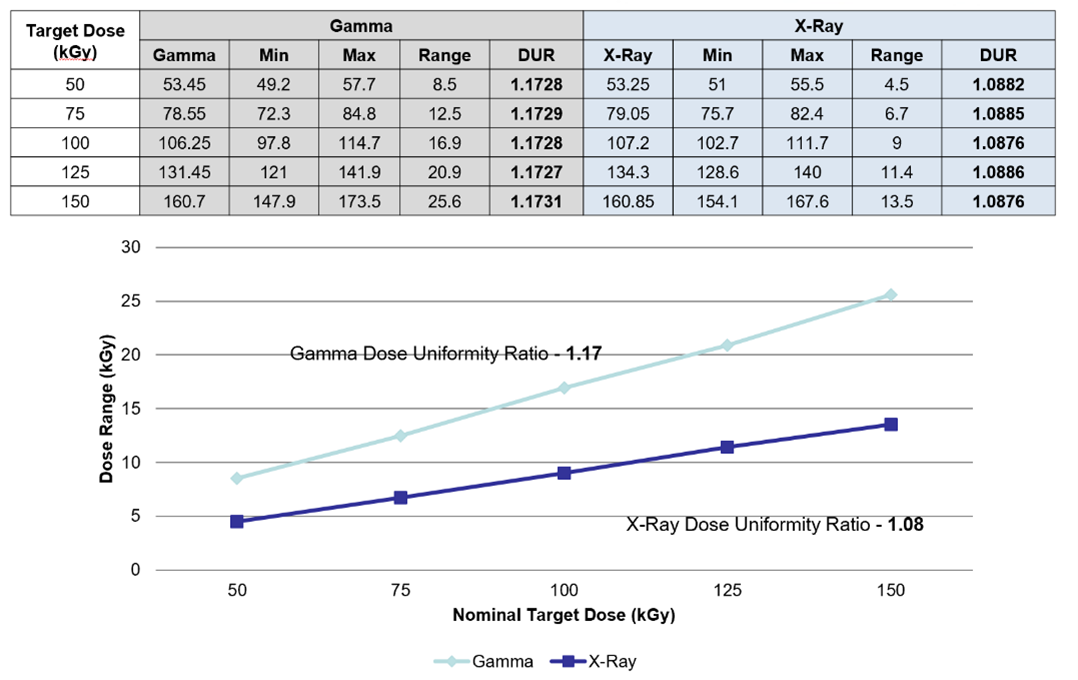
Per Figure 7, using a uniform configuration of UHMWPE, an 8% reduction in DUR was observed by moving from gamma to X-ray. With improved DUR, the dose could be delivered to the product more precisely.
Figure 7: Gamma and X-ray DUR results from the treatment of UHMWPE at target doses of 50 to 150kGy. DUR = dose uniformity ratio; UHMWPE = ultra high molecular weight polyethylene
Case Study 2: Temperature Sensitive Product
In another example, where a temperature-sensitive product was evaluated, a DUR improvement from 1.47 to 1.22 was observed by moving process from gamma to X-ray. This lower DUR (lower maximum dose = less energy absorbed by the product = lower temperature increase) coupled with a higher dose rate resulted in reduced processing time and product temperature.
Case Study 3: Increase of Process Load Volume
As shown in , improved DUR results in more product being processed on the processing unit: In this case, the volume of product per pallet can be more than doubled.
Figure 8: Example of gamma and X-ray pallet configuration. Because of improved DUR, a higher volume of product per pallet can be processed with X-ray. [Final pallet configurations in Gamma and Xray achieved similar DUR’s of 1.18 and 1.21 respectively].
Processing Flexibility
Many gamma facilities operate “shuffle and dwell” operations where the dwell period is a factor of the amount of cobalt present and of the density of the products within the irradiator. In such an operation, products of similar density and dose requirements may be batched to achieve the desired result. Also, the transition effect from one density to another may need to be taken into account. As a consequence, scheduling limitations may be experienced. As explained in other sections of this article, X-ray processing, having the benefit of being incremental and working over a reduced treatment area, results in no significant impact on the pallets neighboring the processed pallet. Subsequently, pallets of different densities but also different target dose can be processed together, offering improved processing flexibility.
Conclusions—Future Opportunities with X-ray
As described in this article, experience thus far with industrial-scale X-ray radiation of healthcare products and materials has yielded some very promising outcomes. For example, as dose uniformity is improved, maximum dose is lowered, resulting in 1) improved economics as more individual products are processed on the processing unit; 2) improvement in processing efficiency and supply chain as more products are processed more quickly; 3) greater material compatibility as lower maximum doses achieve the same requirement of sterility assurance; and 4) improved product outcomes with reduction in temperature during processing. Furthermore, the levels of oxidation observed with X-ray appear improved compared to those seen in gamma at the same dose levels.
As highlighted by Plaček & Bartoníček,[15] the oxidation of polymeric materials is “strongly influenced by the atmosphere in which they are irradiated,” such that the radiation dose required for reaching a particular level of degradation changes with the dose rate. The authors observed that the rate of oxygen diffusion at higher dose rates is insufficient to support the oxidation reactions, contrary to that observed at lower dose rates.15 Impacts such as these require further in-depth investigation. Such investigative work will continue to inform and impart knowledge and further develop the understanding summarized in Table 1.
| Gamma | E-beam | X-ray | |
|---|---|---|---|
| Mode of action | Isotropic Photons
Average Energy 1.25 MeV |
Electron
Typically, 10 MeV energy |
Photons with almost the same direction
90% of photon energy approximately 0.3MeV |
| Largest processing unit | Pallets or boxes | Boxes | Pallets or boxes |
| DUR | Typical dose range achievable for medical device density 25-40 kGy;
Ideal: 25-50 kGy |
Typical minimal dose range achievable need for medical device density 25-50 kGy
Ideal 25-60 kGy |
Typical dose range achievable for medical device density 25-35 kGy.
Ideal: 25-40 kGy |
| Dose rate | A few kGy/h | A few 1000 kGy/h | A few kGy/h to a few hundred of kGy/h |
| Temperature | Depend on design and Cobalt activity
Typically, maximum temperature can go to 45 to 50°C. |
Depends on power
Typically, maximum temperature can go to 50°C. |
Depend on power and design
Typically, maximum temperature can go to 35°C to 40°C. |
As suppliers of X-ray irradiators bring innovations and design concepts to the market, the variances in product flow, energy, power, and dose rate must be understood and evaluated. Regardless of such variances, the opportunity to provide a viable, efficient, and economical alternative to gamma radiation is a reality. This will help address the concerns regarding cobalt supply, transportation, and security highlighted previously.3
Acknowledgements
The authors would like to thank Ms. Emily Craven, cochair of AAMI ST/WG2 on Radiation Sterilization for her valuable review and input to this article. The authors would like to thank and acknowledge the Kilmer Collaboration Modalities group for the collaborative efforts considering and exploring how additional sterilization modalities may be deployed and adopted by the healthcare products manufacturing industry, for the benefit of patient care.
Bibliography
[1] Cleland MR. Electron beam materials irradiators. In: Hamm ME, Hamm RW, Eds. Industrial Accelerators and Their Applications. Singapore: World Scientific Publishing Co Pte Ltd; 2012:87–138.
[2] IAEA 1967. Radiosterilization of medical products, pharmaceutical and biopharmaceuticals. Vienna: International Atomic Energy Agency.
[3] Kroc TK, Thangaraj JCT, Penning RT, Kephart RD. Accelerator-driven Medical Sterilization to Replace Co-60 Sources. Fermi National Accelerator Laboratory: 2017. https://lss.fnal.gov/archive/2017/pub/fermilab-pub-17-314-di.pdf. Accessed June 4, 2020.
[4] Cleland MR, Stichelbaut F. Radiation processing with high-energy X-rays. Radiation Physics and Chemistry. 2013;(84):91–99.
[5] IAEA 2008. Trends in radiation sterilization of health care products. Vienna: International Atomic Energy Agency.
[6] Gamma Irradiation Processing Alliance and International Irradiation Association. A Comparison of Gamma, E-beam, X-ray and Ethylene Oxide Technologies for the Industrial Sterilization of Medical Devices and Healthcare Products. 2017. http://iiaglobal.com/wp-content/uploads/2018/01/White-Paper-Comparison-Gamma-Eb-Xray-and-EO-for-Sterilisation.pdf. Accessed June 4, 2020.
[7] Meissner J, Abs M, Cleland MR, Herer AS, Jongen Y, Kuntz F, Strasser A. X-ray treatment at 5 MeV and above. Radiation Physics and Chemistry. 2000;(57):647–651.
[8] Lambert BJ, Mendelson TA, Craven MD. Radiation and ethylene oxide terminal sterilization experiences with drug eluting stent products. AAPS PharmSciTech 2011;12(4):1116–1126.
[9] ASTM F1980-16:2016. Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices. West Conshohocken, PA: ASTM International.
[10] AAMI TIR17:2017. Compatibility of materials subject to sterilization. Arlington, VA: Association for the Advancement of Medical Instrumentation.
[11] ASTM F2565. Standard Guide for Extensively Irradiation-Crosslinked Ultra-High Molecular Weight Polyethylene Fabricated Forms for Surgical Implant Applications. West Conshohocken, PA: ASTM International.
[12] Michel H, Hartmann H. Qualification of the properties of Highly Crosslinked UHMWPE, to be routinely processed with a 7 MeV X-Ray irradiator. Paper presented at International Meeting on radiation Processing (IMRP) 7-10 Nov 2016, Vancouver (CA) and at Ionizing Radiation and Polymers Symposium (IRAP) 25–30 Sept 2016.
[13] ISO 11139:2018. Sterilization of health care products—Vocabulary of terms used in sterilization and related equipment and process standards. Geneva: International Organization for Standardization.
[14] Grégoire O, Cleland MR, Mittendorfer J, Vander Donckt M, Meissner J. Radiological safety of medical devices sterilized with X-rays at 7.5 MeV. Radiation Physics and Chemistry. 2003;67(2):149–167.
[15] Plaček V, Bartoníček B. The dose rate effect and the homogeneity of radio-oxidation of plastics. Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms. 2001;185(1–4):355–359.