Introduction
This TechTip outlines a practical approach to establishing an appropriate internal PCD (IPCD) to minimize over-processing while still meeting a minimum sterility assurance level (SAL) of 10-6. Historically, industrial practices for choosing IPCDs have leaned towards selection of “the worst of the worst” after comparative resistance ranking data have been generated from sublethal experiments. STERIS proposes a scientifically sound approach aligned to requirements of ISO 11135:2014 by choosing an optimal IPCD based on the sublethal comparative analysis, even when it is not the “worst of the worst” in the ranking. By considering all IPCDs that present a greater challenge than the natural product bioburden in any location on the device, the requirements of the ISO standard can be met without creating an unnecessary and extreme over-challenge to the process.
Background
What do the ISO 11135:2014 requirements say about internal PCDs?
ISO 11135:2014 states, “An internal PCD is used to demonstrate that the required product SAL is achieved.” Therefore, the selection of an appropriate IPCD is a critical decision in the validation of an EO process. The IPCD needs to be demonstrated to be an equal or greater challenge to the process than the bioburden of the product in the most challenging location.
| ISO 11135:2014 | Sterilization of health-care products – Ethylene oxide – Requirements for the development, validation and routine control of a sterilization process for medical devices |
|---|---|
| ISO 14161:2009 | Sterilization of health care products — Biological indicators — Guidance for the selection, use and interpretation of results |
| ISO 10993-7:2008 | Biological evaluation of medical devices — Part 7: Ethylene oxide sterilization residuals |
| ISO 11737-1:2018 | Sterilization of health care products — Microbiological methods – Part 1: Determination of a population of microorganisms on products |
| ISO 11737-2:2009 | Sterilization of medical devices — Microbiological methods — Part 2: Tests of sterility performed in the definition, validation and maintenance of a sterilization process |
| AAMI TIR16:2017 | Microbiological aspects of ethylene oxide sterilization |
What are the requirements for establishing an Internal PCD?
Clause 3.28 of ISO 11135:2014 defines a PCD as an “item designed to constitute a defined resistance to a sterilization process and used to assess performance of the process.” Notes 1 and 2 state “a PCD can be product, simulated product, or other device that is inoculated directly or indirectly… A PCD located within the confines of the product or product shipper case is an internal PCD.”
Section 7.1.6 of ISO 11135:2014 states “It shall be demonstrated that the specified sterilization process is effective in sterilizing the most difficult-to-sterilize location within the product.” This is addressed in the process / product qualification using biological indicators (BIs) which have been placed in an appropriate process challenge device (PCD). The following paragraph provides details on the criteria required for BIs used in the establishment of the sterilization process as outlined in section 8.6 of ISO 11135:2014:
Biological indicators (BIs) used as part of the establishment of the sterilization process shall
a) comply with ISO 11138-2:2006, Clause 5 and 9.5,
b) be shown to be at least as resistant to EO as is the bioburden of product to be sterilized, and
c) be placed within an appropriate PCD.
The appropriateness of the PCD used for process definition, validation or routine monitoring and control shall be determined. The PCD shall present a challenge to the sterilization process that is equivalent or greater than the challenge presented by the natural bioburden at the most difficult to sterilize location within the product.
NOTE: For information on the selection, use and interpretation of biological indicators, see ISO 14161.
The key to the selection of an appropriate IPCD is the demonstration that the IPCD shall present a challenge to the sterilization process that is equivalent or greater than the challenge presented by the natural bioburden at the most difficult to sterilize location within the product.
Three approaches are outlined in D.8.6 of ISO 11135:2014 for establishing the appropriateness of a PCD, of which ‘Approach 2’ is widely used for this purpose. The intent of this approach is to achieve negative growth for all product tests of sterility and survivors of the test microorganism from the BI/PCD. This establishes objective evidence of the comparative challenge of the bioburden of the product, including its most difficult to sterilize location(s), to that of the test microorganism in the BI/PCD.
The comparative resistance of product bioburden and PCD(s) containing a BI with a population of a minimum of 10-6 is illustrated in Figure 1 (below).
Section 7.3.1 of ISO 11135:2014 requires that medical device manufacturers have a system that specifies and maintains the microbiological quality and cleanliness of the product presented for sterilization in a manner that does not compromise the effectiveness of the sterilization process. The manufacturing controls typically result in consistent bioburden levels comprised of microorganisms lower in population and D value than that of biological indicator organism, Bacillus atrophaeus.
Figure 1 illustrates the relative resistance between that of a PCD containing a BI with a minimum population of 10-6 and that of a typical bioburden (shaded area) found on medical devices.
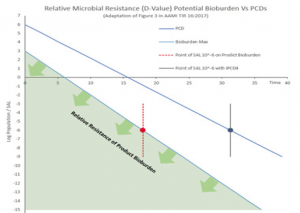
The D value curves above illustrate that the PCD presents a greater challenge to the sterilization process than that of the product bioburden, meeting the requirements set out in section 8.6 of ISO 11135:2014.
Selection of an appropriate PCD based on comparison against the relative resistance of the product bioburden using test of sterility allows manufacturers optimize the EO concentration and dwell when qualifying the required SAL of 10-6. Designing a PCD that presents a greater challenge to the process, for example, by placing a BI in a single, worst case location in the product should be avoided, where possible. Selection of an overly challenging PCD may far exceed the microbiological challenge of the product bioburden. This in turn may in the process requiring additional lethality (EO concentration and / or dwell time) to achieve the required kill in half cycles.
Important Note:
Refer to sections 5.1 and 7 of ISO 11737-2:2009 for the selection of samples and assessment of test method for the Test of Sterility used in this approach.
Summary
How do you select an appropriate IPCD?
- Negative growth from tests of sterility of the product along with positive growth from the BIs in the IPCD demonstrates the appropriateness of an IPCD as it establishes objective evidence that the IPCD presents a greater challenge to the sterilization process than that of the product bioburden, including the most difficult to sterilize locations on the product.
Which IPCD location should be selected for testing?
- Where multiple IPCD locations are demonstrated to be appropriate, the one that is least challenging may be selected based on demonstration of higher resistance to the process than the natural bioburden.
What happens if the most challenging internal PCD is selected?
- Selection of the most challenging IPCD may require increased lethality conditions (e.g. EO concentration, exposure time) to achieve full inactivation in microbiological performance qualification (MPQ) cycles.
Why should “false” challenges to BI placement in an ICPD be avoided?
- When selecting a location to place a BI in an IPCD, the potential to introduce a “false” challenge by occluding or changing the way the product is presented during routine processing should be avoided.
When should process lethality be reduced?
- When there is no growth from both the IPCD and the product tests of sterility, process lethality may be reduced by shortening the dwell time and / or reducing the EO concentration to provide data to support selection of an optimal IPCD.
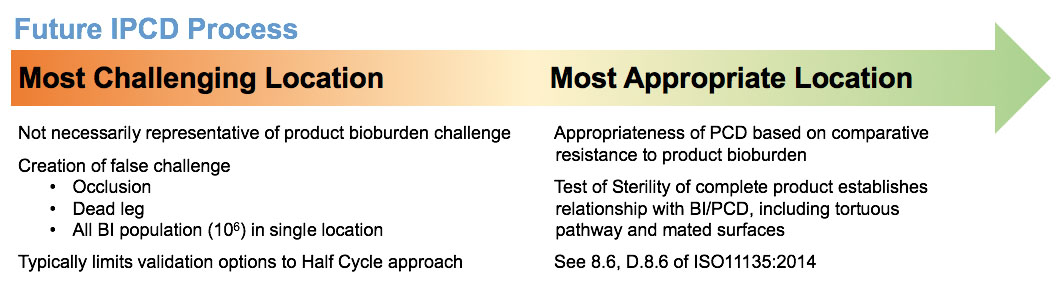
Finally, Figure 2 illustrates the evolution to a practical approach of establishing an appropriate internal PCD (IPCD) to minimize over-processing while still meeting a minimum sterility assurance level (SAL) of 10-6
Figure 2
For further information on the selection of an appropriate PCD, please contact a member of our TechTeam.
Related TechTips
Ethylene Oxide Master File Pilot Program FAQ
Q: What is the Ethylene Oxide (EO) Sterilization Master File Pilot Program? A: In an effort to advance innovation in medical device sterilization with ethylene oxide (EO) and reduce the threat of shortages of EO-sterilized medical devices by providing
Study on the Impact of EO Concentration on Product Residuals
A study was carried out to compare the impact, if any, of EO concentration on product EO residual levels determined in accordance with ISO10993-7.
Sustainable EO® Frequently Asked Questions
As part of our commitment to providing innovative and sustainable solutions, STERIS AST has developed the Sustainable EO® sterilization services program.
Overview Of Sterilization Technology Comparison
Learn more about our global offering, which includes electron beam, gamma, X-ray, and ethylene oxide technologies

