
Microbiology Testing Services
Our microbiology testing services support the sterilization validation and laboratory testing needs of our medical device and pharmaceutical Customers. We provide a single source for every stage of the sterilization design process, from product development through routine processing. Our microbiology laboratories are fully accredited, adhere to the requirements of ISO11135 and ISO11137. View our full list of microbiology testing services below
Antimicrobial Effectiveness
Demonstrates the effectiveness of the preservative system in a product.
Aseptic Processing Qualification and Monitoring
Demonstrates that environmental controls are sufficient to manufacture a drug or therapy by aseptic processing methods
Bioburden Testing
Measures the microbial contamination levels on or in a product.

Biological Indicators
Biological indicator testing and product inoculation.

Cleanroom Testing & Consultancy
Demonstrates the control of viable and non-viable particles in critical areas.

Disinfectant Efficacy Testing
Design and execution of in vitro efficacy studies, and recommendations for the application of qualified disinfectants and sporicides.
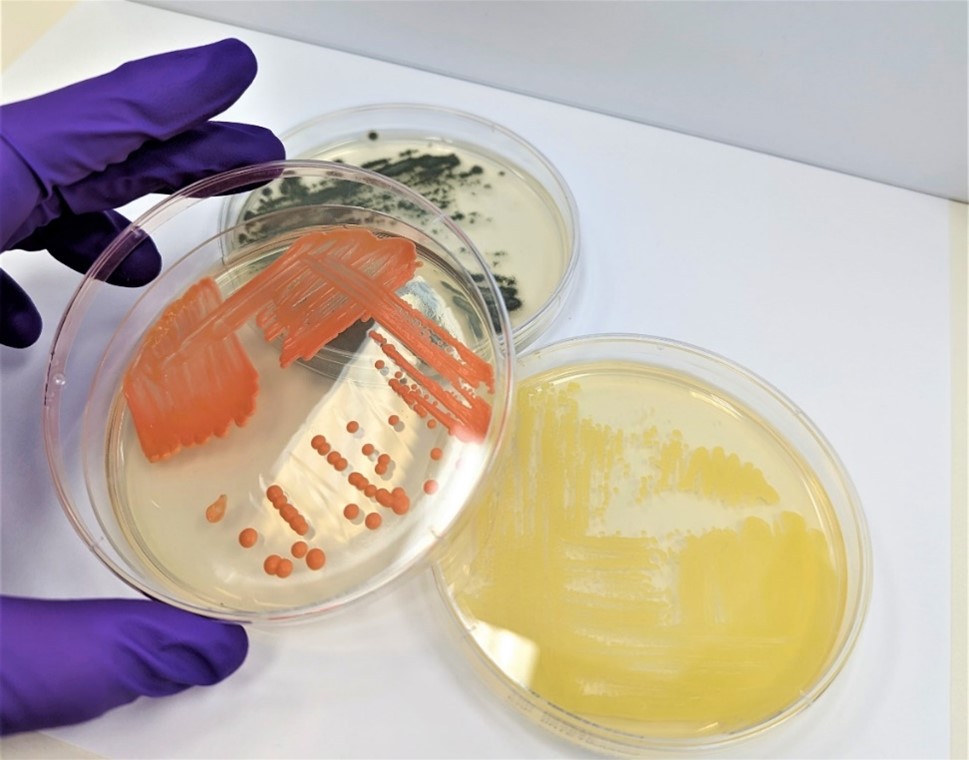
Microbial Identification
Rapid methods for microbial identification in pharmaceutical and medical device manufacturing and packaging environments.

Microbial Limits Testing
Determines whether products comply with an established specification for microbial quality.

Radiation Sterilization Validation Services
Technical support in all phases of the radiation sterilization design process.

Sterility Testing
Demonstrates the effectiveness of a sterilization process to ensure products are free from the presence of viable microorganisms. .

Sterilization Validation
Testing used to determine the minimum sterilization dose required for products making a sterile claim.

Tissue Testing
Tissue and tissue-based testing utilizing dedicated test equipment to process a wide range of tissue samples..


