ISO11137 Method 1 & VD Max Dose Studies
STERIS assists Customers at all levels of sterilization validation for ISO11137 (radiation sterilization) as required for products with a sterility claim.
Working in coordination with the STERIS Applied Sterilization Technologies processing sites and TechTeam®, we provide technical support in all phases of the radiation design process, including technical support, laboratory testing services, product and packaging testing services, and supporting the transfer into routine sterilization.
Our sterilization validation team conducts studies as a single validation or as part of a facility startup or technology transfer either onsite or at STERIS. As part of your complete radiation validation program, we will develop a protocol, assemble all testing and validation data, summarize results and make recommendations in a succinct and comprehensive format. With the proximity and co-operation of the Sterilization teams we ensure this is a single process for the Customer to follow with one point of contact.
STERIS offers:
- A complete service package including initial microbiological method development, verification dose and final sterility testing. The complete sterilization validation is detailed in one report along with the irradiation certificate.
- A range of expertise covering the microbiological testing as well as recommendations and support for ongoing microbiological audits and monitoring. Ensuring our Customers achieve a tailored product/device sterilization solution
- Experience providing sterilization validations for complex or unique products, plus products that are sensitive to irradiation and may require lower irradiation doses.
- Experience in completing Method 2 studies on products which are unable to obtain a sterilization dose through the more common approaches.
- Testing lead times available to suit product submission timelines
- Continuous support, consultancy and updates within the sterilization validation process
ISO 11137: VDMax Study (Irradiation Validation)
The VDMax study (irradiation validation) is the substantiation of the required minimum irradiation dose. The required processing irradiation dose is chosen based on knowledge of the products.
The initial sterilization validation begins by calculating the average bioburden for samples from three different batches/lots of product. Using the irradiation dose tables from ISO 11137/13004, bioburden count is used to calculate the sub-lethal verification dose. The verification dose is applied to ten samples of product in one of our sterilization facilities and then returned to the lab for testing of sterility. If there is 1 or less failures, the validation is passed and the routine processing irradiation dose that was chosen is substantiated.
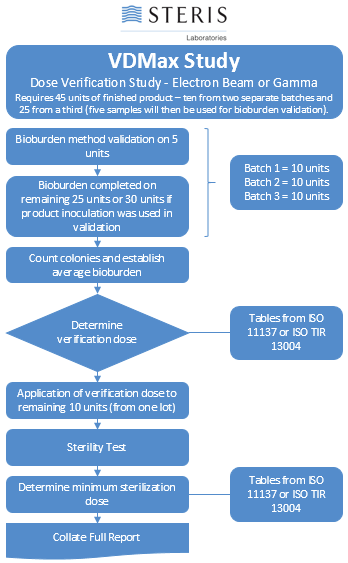
The VDMax study doses are shown in the table along with the maximum allowable bioburden level for each dose.
ISO 11137: Method 1 study (Irradiation Validation)
A Method 1 study is performed to verify a minimum irradiation dose for a particular bioburden level on a product.
The initial sterilization validation is started by calculating the average bioburden for samples from three different batches/lots of product. Using the irradiation dose tables from ISO 11137, bioburden count is used to calculate the sub-lethal verification dose. The verification dose is applied to 100 samples of product in one of our sterilization facilities and then returned to the lab for testing of sterility. If there are two or less failures, the validation is passed and the routine processing irradiation dose is calculated from ISO 11137 based on the verification dose.



