Rigorous Highly Accelerated Life Testing (HALT) prevented telecommunications company from a complete product recall.
Challenge
HALT Testing is a highly aggressive temperature and vibration cycling process which is used very early in the design stage of the product life cycle.
A major telecoms company were performing a series of various tests on a complex and high-value product, which consisted of several large plug-in cards secured to a large backplane. Initially, extensive design verification testing was performed to qualify and validate the product. No problems or failures were found during this test process. Afterward, several complete units were sent to external sites for alpha/beta testing. This type of evaluation lasted for several months and was completed successfully without any failures.
Solution
The next stage was the final qualification where the product was subjected to HALT at the STERIS test laboratory. During the fast ramp temperature excursions from the Upper Operating Limit (UOL) to the Lower Operating Limit (LOL), failures started to occur causing major functional errors.
Failure analysis found that an inner layer etch was defective, opening and closing like an electronic switch. This failure was caused by a manufacturing error in the backplane and exposed by HALT during the high to low-temperature fast ramp rates causing the inner layer etch to expand and contract. When the product was brought back to ambient temperature it performed correctly.
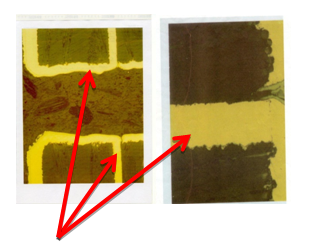
Arrows point to breaks on inner layer PCB etch due to insufficient solder caused during manufacturing process. These flaws were exposed by STERIS’s HALT testing.
Results
The result was a major engineering change on several hundred backplanes. If the product had not been exposed to a HALT, it would have been shipped with the latent defect and possibly resulting in immediate field failures a total product recall. The company subsequently acknowledged that the testing could have saved millions of dollars.
Related TechTips
Product and Packaging Testing FAQ
Product and Packaging Testing Frequently Asked Questions (FAQ) Q1: Per ISO and ASTM standards, is there a recommendation on when a transportation simulation should be performed on aged samples (i.e. after accelerated aging/real-time aging or shelf-life
Reusable Medical Device Cleaning Validation: Soiling Considerations
Background The reusable medical device cleaning process is the process of physically removing contaminants from a device to ensure the safety and effectiveness at the point of use. The validation of the cleaning process, including relevant soils and so
Microbiology Testing FAQ
Microbiology Testing for Sterilization Frequently Asked Questions (FAQ) Q1: What actions need to be performed if bioburden test results come back significantly elevated compared to the product’s historical/trend data? Answer: Routine monitoring of prod
A Guide to Determining the Most Appropriate Distribution Simulation for a Medical or Pharmaceutical Device
Introduction The packaging of sterile medical and pharmaceutical devices needs to ensure that the product shall function as intended and remain sterile to the point of use, from both a patient safety and regulatory requirement perspective. As part of p
